哮喘和慢性阻塞性肺疾病(COPD)是伴随粘液分泌亢进、气道高反应的慢性气道炎症性疾病。目前,关于病毒是否参与了这些疾病的发展,是存在争议的问题。最近,Kim等通过病毒感染建立存在杯状细胞化生、气道高反应的小鼠疾病模型,对此作了进一步的观察。
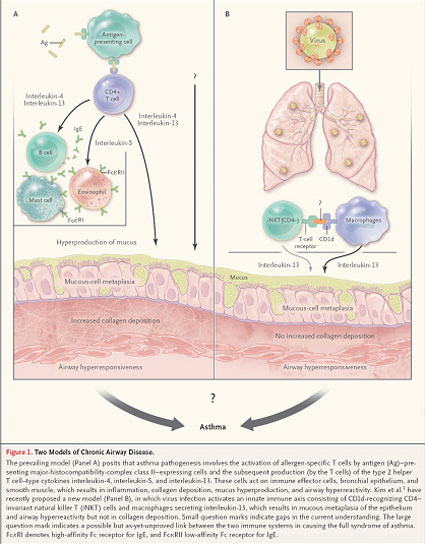
既往大多认为,CD4+ T淋巴细胞识别结合主要组织相容性复合物(major histocompatibility- complex ,MHC)Ⅱ类分子,启动免疫应答,是参与哮喘发生的重要机制。然而,Kim等研究发现,可以在MHC I类分子(供CD8+ T淋巴细胞识别)和MHC Ⅱ类分子(供CD4+ T淋巴细胞识别)缺失的小鼠中观察到慢性气道疾病的发生----予病毒感染小鼠,启动免疫反应,活化CD4+固定的自然杀伤T细胞(invariant natural killer T ,iNKT)表面T细胞受体,识别巨噬细胞表面CD1d分子,分泌IL-13,引起粘膜上皮粘性化生及发生气道高反应,但没有粘膜下的胶原沉积(图1)。Kim等研究不但证实CD4− iNKT在病毒感染诱发的气道高反应的重要地位,而且也观察到敲除CD4+ iNKT,并不影响哮喘小鼠模型的建立。
IL-13目前已成为研究哮喘治疗的重要靶点,Kim等研究充分显示抗IL-13拮抗剂的应用价值。尽管干扰iNKT参与的促炎症反应和免疫调节可能会增加自身免疫疾病发生的危险,但针对哮喘和COPD异常免疫反应进行免疫治疗,仍是十分有效的方法。
(张清玲 深圳市第二人民医院呼吸内科 518039 摘译)
(N Engl J Med ,2008 November;359:2062-2064.)
Ratko Djukanović, M.D., and Stephan D. Gadola, M.D. Virus Infection, Asthma, and Chronic Obstructive Pulmonary Disease. N Engl J Med ,2008 November;359:2062-2064.
Virus Infection, Asthma, and Chronic Obstructive
Pulmonary Disease
Ratko Djukanović, M.D., and Stephan D. Gadola, M.D.
Both asthma and chronic obstructive pulmonary disease (COPD) are characterized by chronic inflammation of the airways which is accompanied by increased mucus production and airway hyperreactivity. It has long been speculated but not clearly shown that viruses are involved in the development of these diseases. Kim and colleagues1 recently provided grist for this mill with their observation of chronic goblet-cell metaplasia and airway hyperreactivity in mice after virus infection.
According to the prevailing paradigm, major histocompatibility- complex (MHC) class II–dependent CD4+ T lymphocytes, through their response to stimulation by environmental allergens, are key to the pathogenesis of human asthma (Fig. 1). The data described by Kim et al found that chronic airway disease can develop in the absence of both MHC class I–dependent CD8+ T cells and MHC class II–dependent CD4+ T cells. Kim et al. have recently proposed a new model (Panel B), in which virus infection activates an innate immune axis consisting of CD1d-recognizing CD4−invariant natural killer T (iNKT) cells and macrophages secreting interleukin-13, which results in mucous metaplasia of the epithelium and airway hyperreactivity but not in collagen deposition. They not only identified CD4− invariant natural killer T cells as key players in virus-induced airway hyperreactivity but also observed that the depletion of CD4+ invariant natural killer T cells had no effect in the model they used.
Interleukin-13 is widely viewed as a potential target for new therapy in patients with established asthma, so this study adds to the body of evidence supporting the use of anti–interleukin-13 therapies. Although interfering with the fine balance between proinflammatory and regulatory processes modified by invariant natural killer T cells may increase the risk of inducing autoimmunity, targeting such cells may well prove to be effective in both asthma and COPD.